Lakes around the world are turning brown. This is both good and bad. Browning is a sign of recovery from acid rain, but it’s also a symptom of climate change and development. Discover why lakes are browning, consequences for lake health, and how you can help monitor water quality and contribute to global datasets.
Lakes are connected to their watershed
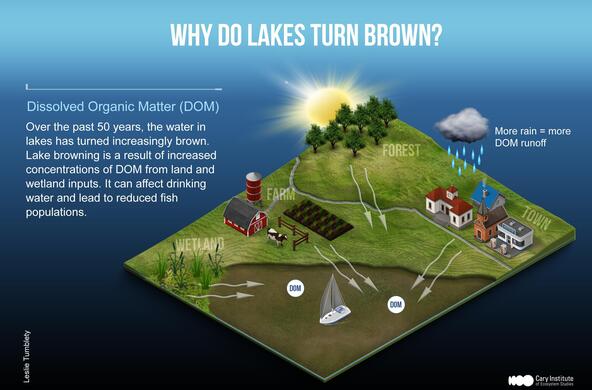
Plant matter and pollutants from the landscape flow into lakes. Since lakes are often the lowest point in the watershed, they are reliable indicators of watershed health. Landscapes that are heavily forested deliver high amounts of dissolved organic matter (DOM) from decaying leaf litter and soil. Agricultural runoff contributes excess nutrients which fuel algal growth. As landscapes change, so does water clarity and lake chemistry. Monitoring lakes can provide early warning of broader changes across the landscape.

Why are lakes browning?
Lakes turn brown due to an increase in dissolved organic matter. DOM is transported from the watershed and soil into lakes. As the land recovers from acidification, more organic material is released in the soil and flows into lakes. Browning can also occur due to climate change; increased precipitation and storms can increase DOM runoff into lakes.
Why does lake browning matter?
Browner lakes are less clear, which impacts many lake features. Light penetrates shallower in brown waters, so surface waters heat up faster in browner lakes. Reduced water clarity can depress oxygen levels and algal productivity, with implications for aquatic organisms and the lake food web.
Browning is both good and bad. It is a sign of recovery from acid rain, but it’s also a result of our activities, like development and climate change. Perceptions of browning are also mixed. Life returning to lakes and watersheds is good, but many people prefer to recreate in clear lakes. And lakes that are used for drinking water may be at risk due to changes in lake processes caused by browning. Understanding these effects can inform smart lake management.

Browning may be a signal of ecosystem recovery
Decades of industrial pollution and acid rain caused water bodies in the northeastern US to become strongly acidic. Through national policies like the 1990 Clean Air Act Amendments, industrial polluters were forced to clean up their act. Due to reduced air pollution, both rain and lakes became less acidic. As a result, lake pH increased.
Acid rain caused many lakes to become clear. This is because acidic conditions kill phytoplankton, which float in the water column and reduce clarity. As lakes and the landscape recover and become less acidic, more organisms can survive and more dissolved plant matter flows through the watershed into lakes. Organic matter is brown, which is why lake browning is associated with acid rain recovery.
You can help monitor lake health
Water clarity is an important indicator of lake health. The most common way to measure water clarity is with a Secchi disk (pictured below/above), a tool that has been used for over a century. Watch this video to learn how you can create your own Secchi disk, contribute data to lake research, and help protect lakes for future generations.
Have a question? You can contact a lake scientist:
Dr. Jonathan Stetler stetlj2@rpi.edu // jtstetler@outlook.com
Jonathan Stetler, now a lecturer at RPI, produced this brochure while working as an NSF intern with Cary's Chris Solomon and the Cary Communications Office.
Want to learn more? Explore the resources below:
Invasive species, water clarity, and population changes are some of the challenges lake associations are facing. The Lake Resilience and Systems Thinking Resource Hub includes videos and downloads to help guide discussions about planning for a resilient lake.
Resources for lake associations and local stakeholders have been developed to help with lake management planning and inform lake residents and lake association members on steps they can take to protect lake health.
Jane, S. F., Hansen, G. J. A., Kraemer, B. M., Leavitt, P. R., Mincer, J. L., North, R. L., Pilla, R. M., Stetler, J. T., Williamson, C. E., Woolway, R. I., Arvola, L., Chandra, S., DeGasperi, C. L., Diemer, L., Dunalska, J., Erina, O., Flaim, G., Grossart, H.-P., Hambright, K. D., … Rose, K. C. (2021). Widespread deoxygenation of temperate lakes. Nature, 594(7861), 66–70. https://doi.org/10.1038/s41586-021-03550-y
Leach, T. H., Winslow, L. A., Hayes, N. M., & Rose, K. C. (2019). Decoupled trophic responses to long‐term recovery from acidification and associated browning in lakes. Global Change Biology, 25(5), 1779–1792. https://doi.org/10.1111/gcb.14580
Monteith, D. T., Stoddard, J. L., Evans, C. D., de Wit, H. A., Forsius, M., Høgåsen, T., Wilander, A., Skjelkvåle, B. L., Jeffries, D. S., Vuorenmaa, J., Keller, B., Kopácek, J., & Vesely, J. (2007). Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature, 450(7169), 537–540. https://doi.org/10.1038/nature06316
Solomon, C. T., Jones, S. E., Weidel, B. C., Buffam, I., Fork, M. L., Karlsson, J., Larsen, S., Lennon, J. T., Read, J. S., Sadro, S., & Saros, J. E. (2015). Ecosystem Consequences of Changing Inputs of Terrestrial Dissolved Organic Matter to Lakes: Current Knowledge and Future Challenges. Ecosystems, 18(3), 376–389. https://doi.org/10.1007/s10021-015-9848-y
Stetler, J. T., Knoll, L. B., Driscoll, C. T., & Rose, K. C. (2021). Lake browning generates a spatiotemporal mismatch between dissolved organic carbon and limiting nutrients. Limnology and Oceanography Letters. https://doi.org/10.1002/lol2.10194
Acknowledgements
Funding for this work was provided by the National Science Foundation (grant #1754265).